Calculate the concentration of h2so4 in the undiluted solution – In chemistry, understanding the concentration of solutions is crucial. This article explores the concept of concentration, specifically focusing on calculating the concentration of sulfuric acid (H2SO4) in undiluted solutions. We will delve into the significance of concentration, various units of measurement, and the step-by-step process of calculating concentration using titration methods.
This guide provides a comprehensive overview of concentration determination, empowering readers with the knowledge and techniques to accurately measure and analyze the concentration of H2SO4 solutions.
Sulfuric Acid Concentration: Understanding and Measurement: Calculate The Concentration Of H2so4 In The Undiluted Solution
Sulfuric acid (H2SO4) is a highly corrosive and reactive acid widely used in various industrial processes. Understanding its concentration is crucial for its safe handling, storage, and application.
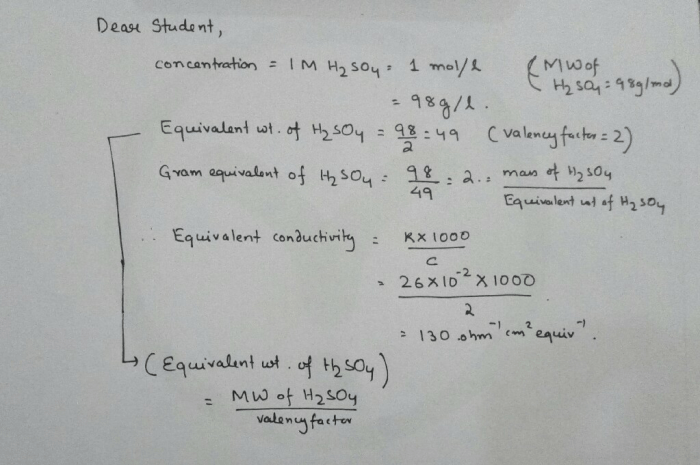
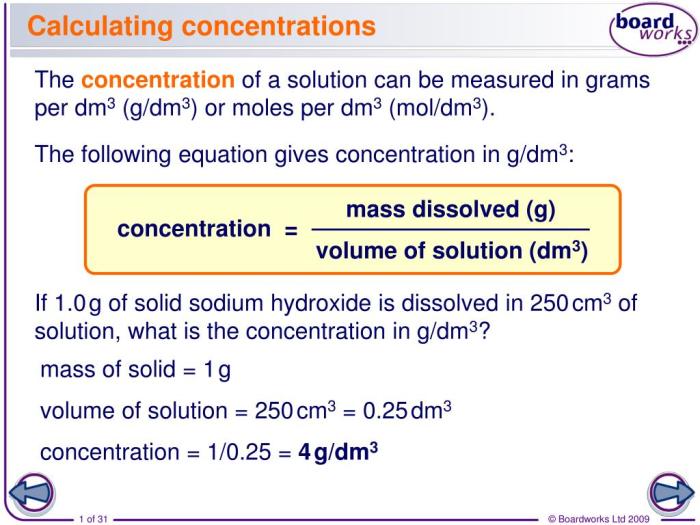
In chemistry, concentration refers to the amount of solute dissolved in a solvent. It can be expressed in different units, such as molarity (M), molality (m), or mass percent (%). Molarity is commonly used to represent the concentration of H2SO4.
Calculating Concentration of Undiluted H2SO4 Solution, Calculate the concentration of h2so4 in the undiluted solution
To calculate the concentration of H2SO4 in an undiluted solution, follow these steps:
- Obtain the density of the solution:Measure the density of the undiluted H2SO4 solution using a pycnometer or other appropriate method.
- Determine the mass of the solution:Weigh a known volume of the undiluted solution using an analytical balance.
- Calculate the mass of H2SO4:Multiply the mass of the solution by the mass percent of H2SO4 in the solution.
- Convert mass to moles:Divide the mass of H2SO4 by its molar mass (98.08 g/mol).
- Calculate the molarity:Divide the moles of H2SO4 by the volume of the solution in liters.
Formula:
“`Molarity (M) = (Mass of H2SO4 in grams) / (Molar mass of H2SO4) / (Volume of solution in liters)“`
Questions Often Asked
What is the importance of concentration in chemistry?
Concentration plays a vital role in chemistry as it determines the amount of solute present in a given volume of solution. It affects the properties and reactivity of the solution, influencing factors such as reaction rates, solubility, and colligative properties.
How does titration help in measuring concentration?
Titration is a technique used to determine the concentration of a solution by reacting it with a solution of known concentration. By monitoring the reaction progress and using stoichiometric calculations, the concentration of the unknown solution can be accurately determined.