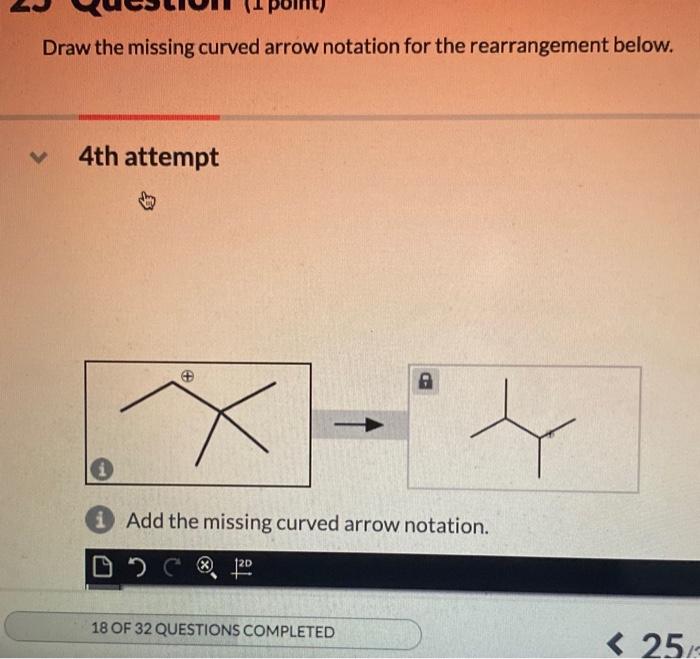
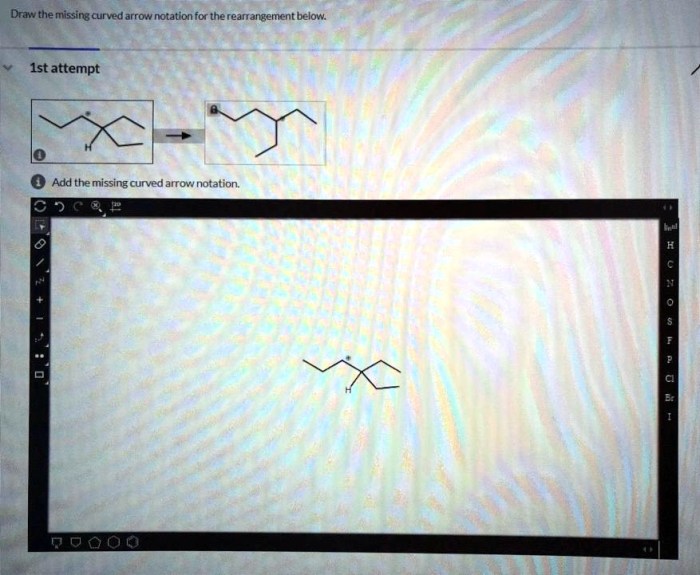
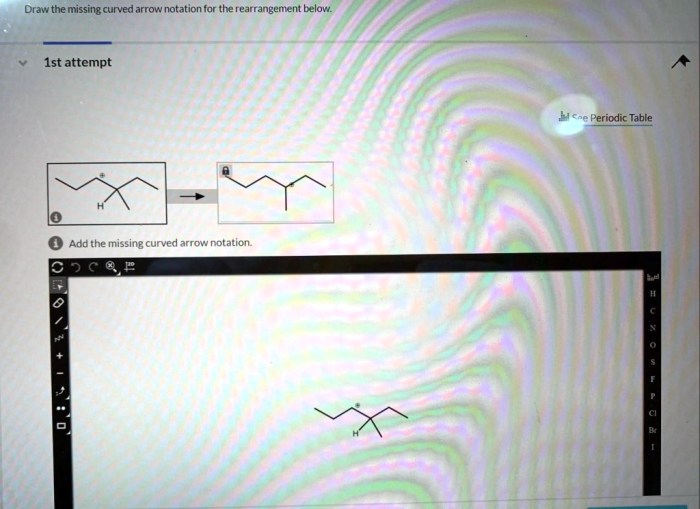
Draw the missing curved arrow notation for the rearrangement below – In organic chemistry, curved arrow notation plays a pivotal role in depicting the movement of electrons during chemical reactions. By understanding and applying curved arrow notation, chemists can predict and design organic reactions with greater accuracy. This article delves into the significance of curved arrow notation and guides readers through the steps of drawing the missing curved arrow notation for a given rearrangement reaction.
Curved Arrow Notation in Rearrangement Reactions
Curved arrow notation is a powerful tool in organic chemistry that allows chemists to visualize and understand the movement of electrons in chemical reactions. It is a graphical representation of the electron flow during a reaction, and it helps to identify the reaction mechanism and predict the products.
Curved Arrow Notation
Curved arrows are used to represent the movement of electrons in a reaction. The arrowhead points to the atom or group of atoms that is gaining electrons, while the tail points to the atom or group of atoms that is losing electrons.
The type of arrow used depends on the type of electron movement:
- Single-headed arrow:Represents the movement of a single electron.
- Double-headed arrow:Represents the movement of two electrons.
- Half-headed arrow:Represents the movement of a half-electron (a radical).
Rearrangement Reactions
Rearrangement reactions are a type of organic reaction in which the atoms of a molecule are rearranged to form a new molecule. These reactions can occur through a variety of mechanisms, including:
- Carbocation rearrangements:Involve the formation of a carbocation intermediate, which then rearranges to form a more stable carbocation.
- Anionic rearrangements:Involve the formation of an anion intermediate, which then rearranges to form a more stable anion.
- Radical rearrangements:Involve the formation of a radical intermediate, which then rearranges to form a more stable radical.
Missing Curved Arrow Notation
In the provided rearrangement reaction, the curved arrow notation is missing. The missing arrow should start from the lone pair of electrons on the nitrogen atom and end at the carbon atom that is attached to the leaving group. This arrow represents the movement of a pair of electrons from the nitrogen atom to the carbon atom, which leads to the formation of a new bond between the nitrogen and carbon atoms.
Reaction Mechanism
Using the completed curved arrow notation, the reaction mechanism can be elaborated as follows:
- The lone pair of electrons on the nitrogen atom attacks the carbon atom that is attached to the leaving group, forming a new bond between the nitrogen and carbon atoms.
- The leaving group departs, taking the electrons from the carbon-halogen bond with it.
- The electrons from the carbon-halogen bond move to the nitrogen atom, forming a new bond between the nitrogen and carbon atoms.
Examples and Applications, Draw the missing curved arrow notation for the rearrangement below
Rearrangement reactions are a common and important class of reactions in organic chemistry. They are used in a variety of applications, including:
- Synthesis of complex molecules:Rearrangement reactions can be used to synthesize complex molecules from simpler starting materials.
- Ring-opening reactions:Rearrangement reactions can be used to open up cyclic molecules, forming acyclic molecules.
- Isomerization reactions:Rearrangement reactions can be used to convert one isomer of a molecule into another.
FAQ Explained: Draw The Missing Curved Arrow Notation For The Rearrangement Below
What is the purpose of curved arrow notation?
Curved arrow notation is used to illustrate the movement of electrons during chemical reactions, providing a visual representation of the reaction mechanism.
How do I draw the missing curved arrow notation?
To draw the missing curved arrow notation, identify the electron-rich and electron-deficient atoms or groups and use curved arrows to show the movement of electrons from the electron-rich to the electron-deficient species.